80 orphan drugs (those drugs intended to treat diseases so rare that sponsors are reluctant to develop them under usual marketing conditions) were approved in the USA in 2017, a record year and more than double the 40 approved in 2016.

Orphan drug spending grew from 5 to 10% of total market spending in the US between 1997 and 2017, while the wider designation of specialty drugs (those classified as high-cost, high complexity and/or high touch) skyrocketed from 12% of total market spending in 1997 to 43% in 2017.
Orphan drug volume and share of total drug volume in the USA between 1993 and 2017.
Spending on orphan drugs by orphan and non-orphan indications in the US between 1992 and 2017. Spending on non-orphan drugs remained relatively static between 2015 and 2017, while spending on orphan drugs has risen from USD 35 billion to USD 50 billion.
A factsheet on orphan drugs in context in the USA.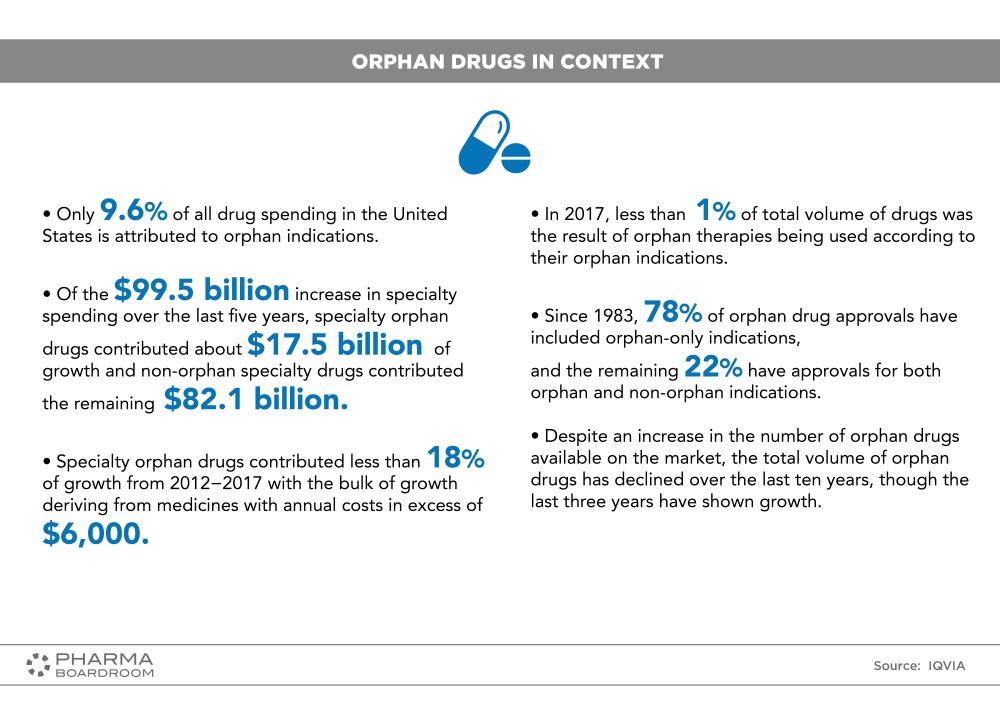

Orphan drug spending grew from 5 to 10% of total market spending in the US between 1997 and 2017, while the wider designation of specialty drugs (those classified as high-cost, high complexity and/or high touch) skyrocketed from 12% of total market spending in 1997 to 43% in 2017.

Orphan drug volume and share of total drug volume in the USA between 1993 and 2017.

Spending on orphan drugs by orphan and non-orphan indications in the US between 1992 and 2017. Spending on non-orphan drugs remained relatively static between 2015 and 2017, while spending on orphan drugs has risen from USD 35 billion to USD 50 billion.

A factsheet on orphan drugs in context in the USA.







