ANSM – Agence Nationale de Sécurité des Médicaments et des Produits de Santé
(National Agency for the Safety of Drugs and Health Products)
Key People:
Dominique Martin, CEO
 The National Agency for the Safety of Medicines and Health Products (ANSM) was created by the government in 2011 for strengthening the safety of medicines and health products in France.
The National Agency for the Safety of Medicines and Health Products (ANSM) was created by the government in 2011 for strengthening the safety of medicines and health products in France.
A public institution under the supervision of the Ministry of Health, the ANSM is financed by a grant for public service charge received from the State, amounting to € 137 million for 2016.
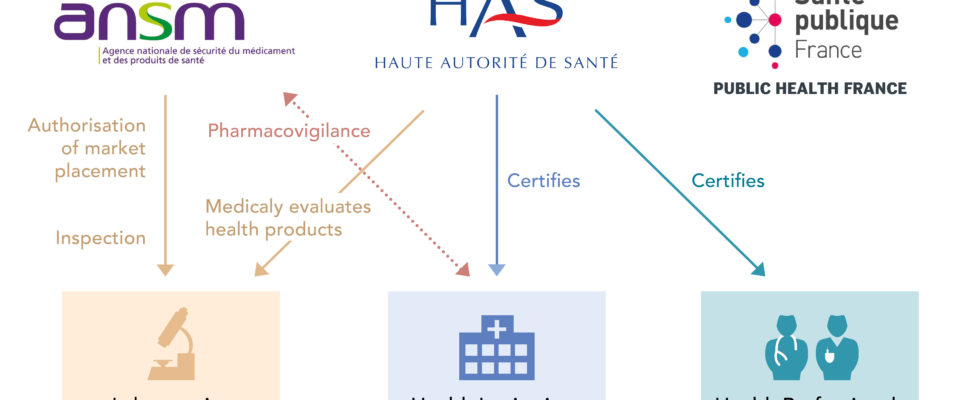
Missions & Responsibilities
- Issue marketing authorizations (MAs) for medicines.
- Monitor and evaluate incidents, adverse events and the risks of incidents or adverse effects related to health products after they have been placed on the market for the to prevent them from happening again.
- Perform laboratory checks on samples of batches of products already present on the market.
- Conduct inspections for all health products under its jurisdiction, including understood internationally. This activity applies throughout the life cycle of health products, from the inspection of raw materials, clinical trials to the inspection of vigilance systems.
- The ANSM, which takes every year more than 80 000 decisions to promote innovation and to guarantee the safety of patients, has a duty to inform and explanation to the population.
Read our recent interview with Dominique Martin.
HAS Haute Autorité de Santé
(The High Authority of Health)
Key People:
Dominique Le Guludec, President
 The Haute Autorité de Santé (HAS) is an independent public authority created by the government in 2004. Since 1 April 2018, its scope has expanded to social and medico-social fields with the integration of the National Agency for the Evaluation and Quality of Social and Medico-Social Institutions and Services (ANESM).
The Haute Autorité de Santé (HAS) is an independent public authority created by the government in 2004. Since 1 April 2018, its scope has expanded to social and medico-social fields with the integration of the National Agency for the Evaluation and Quality of Social and Medico-Social Institutions and Services (ANESM).
It aims to provide people with long-term and equitable access to appropriate, safe and effective care and support.
Missions & Responsibilities
- evaluate health products for reimbursement
- recommend good practices to health, social and medico-social professionals, recommend public health policies
- measure and improve the quality of care in hospitals and clinics, accompaniments in social and medico-social institutions
Santé Publique France
(Public Health France)
Key People:
François Bourdillon, Director General
Santé Publique France serves the population in all aspects of public health based on scientific knowledge, data, and information.
It supports the government and society in improving the health and well-being of the population and applies a population-based approach with the objective of reducing social health inequalities in all areas of public health: Infectious diseases; Non-infectious diseases; Environmental health; Occupational health.
The agency is present throughout the national territory with regional units, including overseas territories, and works in partnership with the Regional Health Agencies (ARS).
Missions & Responsibilities
- Analyze up-to-date knowledge and data on the determinants of health and risk factors.
- Provide decision makers at all levels with independent evidence-based guidance and recommendations. It will be supported by expert committees.
- Propose measures to health authorities to protect the population from health threats.
- Develop evidence-based interventions for prevention and health promotion.
- Contribute to preparedness and management of health crisis, and provide support for the implementation of response plans.
See Below to learn more about government sector organisations in France:
1.French Governmental Organisations and Administrations
2. French Social Security Organizations
3. French Public Authorities and Government Agencies
4. French Parliamentary Bodies
5. French Representative Organisations Forums of the Pharmaceutical Industry